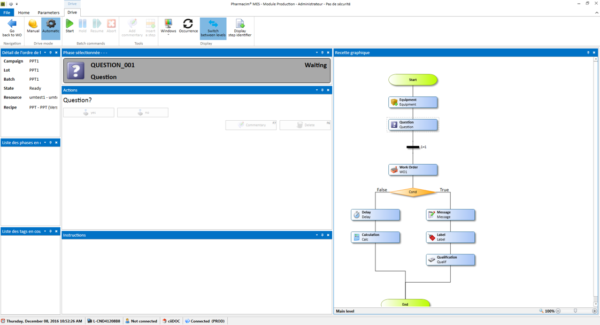
Record validations are very time-consuming for the Quality teams at the end of the batch, Courbon Software has created CiiDOC, a user-friendly solution allowing to take a step toward digitizing the batch file. CiiDOC transforms the batch paper record into a smart document in which the different operators fill in…Read more
New Latin American countries using our Products